

Part 1 of 3 Complete equation: Part 2 of 3 Tonic equation: Part 3 of 3 Net ionic equation: 0-0 0 X Do. Be sure to include physical states of matter and simplify the balanced equation by reducing coefficients when necessary. Question: Provide the complete, balanced reaction, the ionic equation, and the net ionic equation for HCl (aq) + LiOH (aq). Choose an ion from group 2 and react it with phosphate Write a balanced ionic equation and identify the product if a reaction occurred. Write a balanced ionic equation and identify the product if a reaction occurred, C.
Choose an ion from group and react it with phosphate.
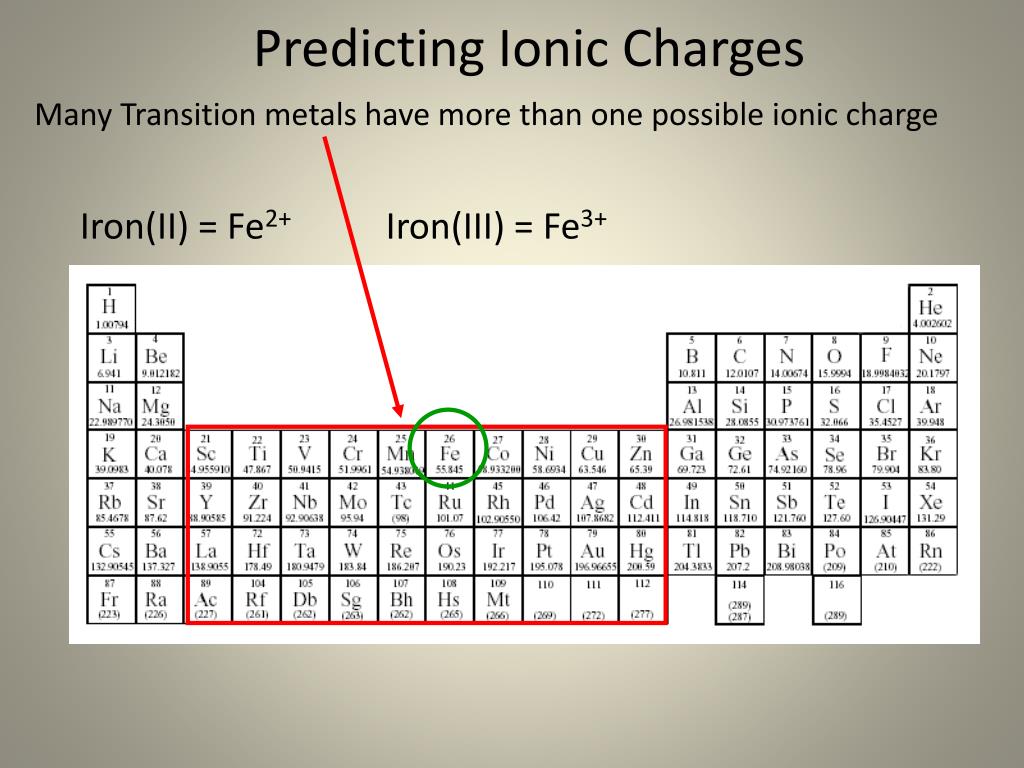
IRON III SULFIDE CHARGE PLUS
Balancing by charge means making sure that the overall charge is the same on both sides of the equation.Magnesium ion plus sulfide ion 6. Balancing by mass means ensuring that there are equal masses of each element on the product and reactant sides. florida lotto wednesday numbers April 15th, 2018 - Net Ionic Equation Worksheet Answers Write balanced molecular ionic and net ionic equations NIE for each of the following reactions Assume all reactions 9 2 Naming And Writing Formulas For Ionic CompoundsNet ionic equations must be balanced by both mass and charge.
Al (s)+NO2− (aq)→NH3 (aq)+AlO2− (aq) Express your answer as a net ionic equation including phases. Enter a balanced net ionic equation for the following reactions in basic solution: ClO− (aq)+MnO2 (s)→Cl− (aq)+MnO4− (aq) Express your answer as a net ionic equation including phases.
IRON III SULFIDE CHARGE HOW TO
How to Write a Net Ionic Equation There are three steps to writing a net ionic equation: Balance the chemical equation.Chemistry. Whether you need help with a product or just have a question, our customer support team is always available to lend a helping hand.Balance the reaction of AgNO3 + H2O = Ag + O2 + HNO3 using this chemical equation balancer! Get support from expert tutors Our expert tutors can help you with any subject, any time.The net ionic equation for the reaction that results from mixing 1 M HCl and 1 M NaOH is: H + (aq) + OH - (aq) → H 2 O (l) The Cl - and Na + ions do not react and are not listed in the net ionic equation. Explain, in your own words, how this second reaction affects the.
IRON III SULFIDE CHARGE FULL
Ba²⁺ (aq) + 2NO₃⁻ (aq) + 2Na⁺ (aq) + 2OH⁻ (aq) → Ba²⁺ (aq) + 2OH⁻ (aq) + 2Na⁺ (aq) + 2NO₃⁻ (aq) ….(5) Cancel out the spectator ions, that is, similar ions on both sides of equation (5) as shown below to get the net ionic equation, The net ionic equation for the reaction you get mixing 1 M HCl and 1 M NaOH is: H + (aq) + OH - (aq) → H 2 O (l) Even though sodium and chlorine exist in the …Which of the following is a balanced net ionic equation for the reaction between hydrochloric acid and potassium hydroxide? H+(aq) + OH−(aq) → H2O(l) Identify the spectator ion(s) in the following reaction: Zn(s) + Cu(NO3)2(aq) → Cu(s) + Zn(NO3)2(aq) NO3-(aq) only When writing an ionic equation, how would Mg(NO3)2(aq) be represented?Net ionic equation calculator is an online tool to get an equation that depicts only the molecules or ions that are actively involved in the Testimonials This app make me understand how math work and solve my homework, best app for learning how to figure out questions that confuse you :), this is great it is very use full but it dosent give you all the … msn hotmail login Net ionic equation calculator is an online tool to get an equation that depicts only the molecules or ions that are actively involved in the Testimonials This app make me understand how math work and solve my homework, best app for learning how to figure out questions that confuse you :), this is great it is very use full but it dosent give you all the …Write the balanced net ionic equation for the reaction of Cl' and AgNO3. Balanced net ionic equations Write equation (4) in the form of the complete net ionic equation, as shown below.


 0 kommentar(er)
0 kommentar(er)
